Med-Biopharm currently exports to over 12 countries with over 100 International Product Registrations (still expanding), we are emerging as a major potential Indian Pharmaceuticals manufacturer. Streamlining the export vision at Med-Biopharm, a dedicated Export regulatory department team has been set up for complying with international audits and providing complete export documentation including COPP and dossier preparations as per CTD format. Operating in total compliance with c-GMP norms, Med-Biopharm’s manufacturing unit has been granted certifications by many regulatory Agencies.
Our Branded and Generic medicines enjoy steady demand in following Countries :-
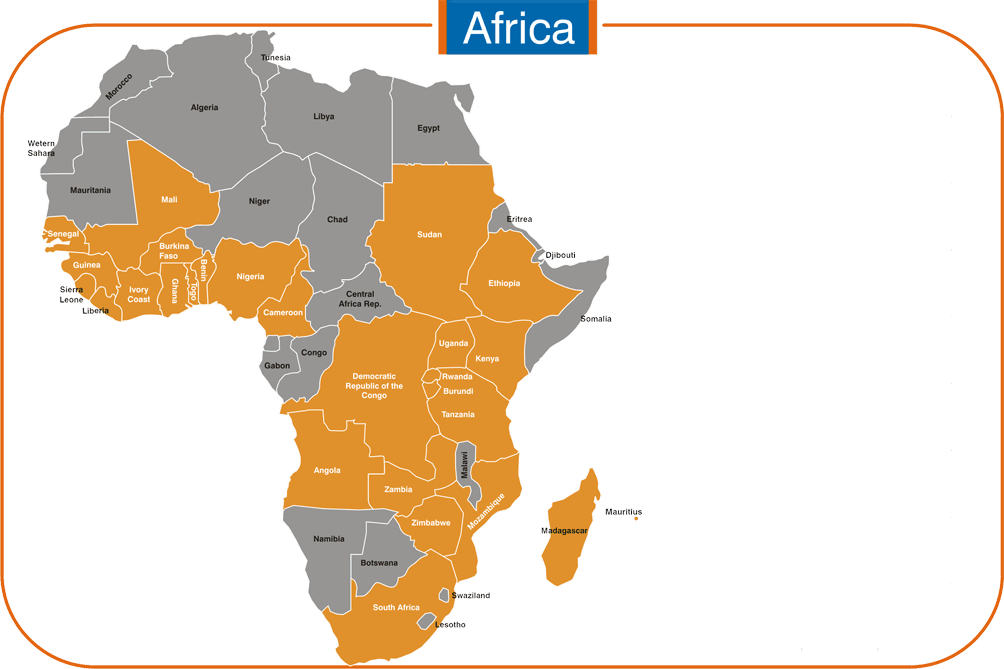
Africa: Cameroon, Nigeria, Gabon & Ivory Coast.
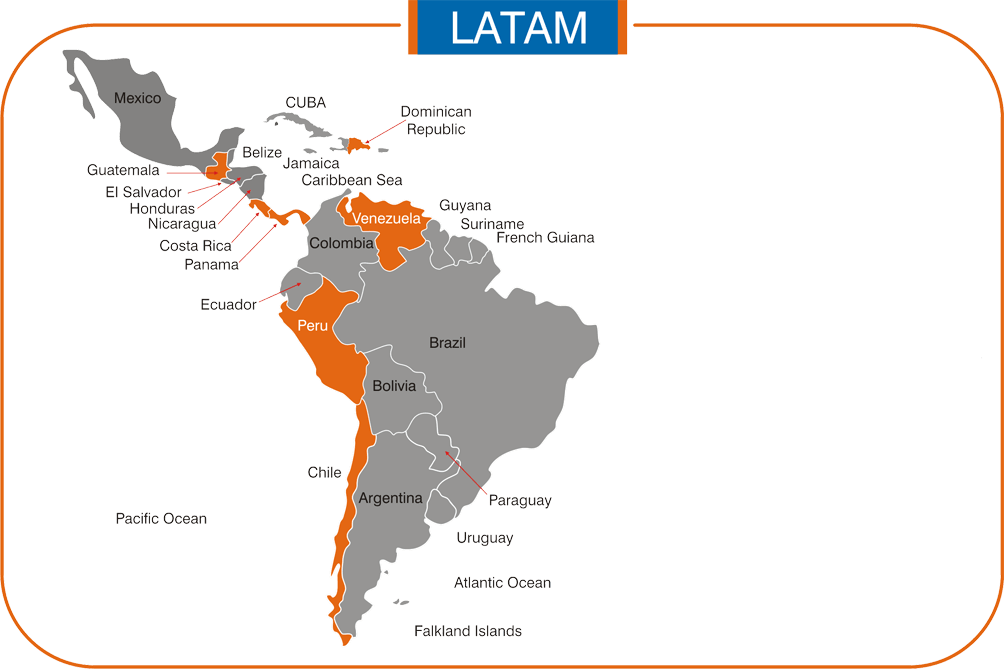
North & South America: Costa Rica, Ecuador, Panama & Guatemala.
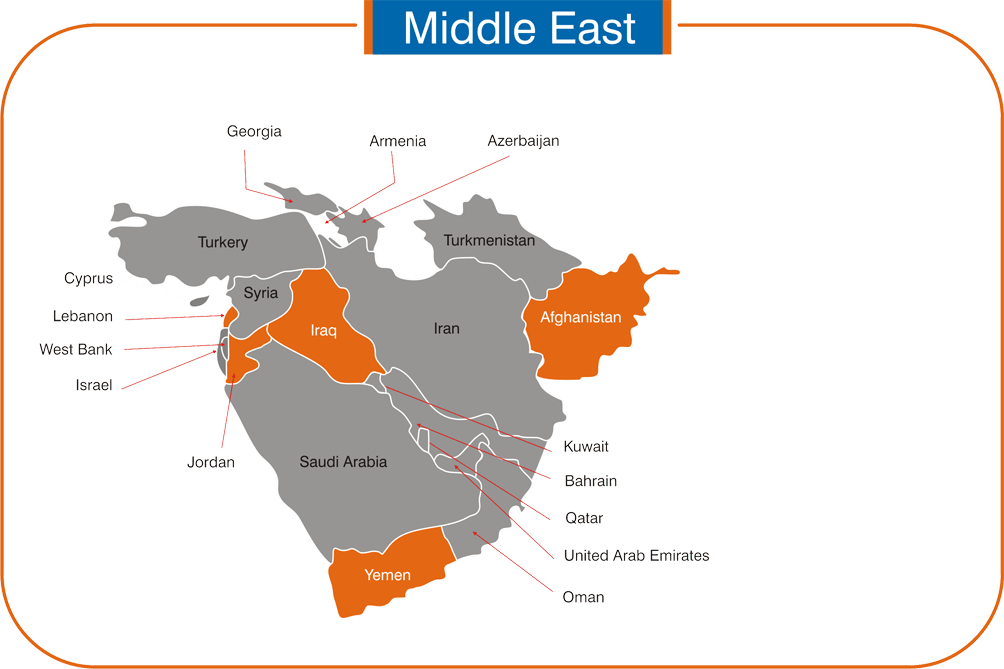
ROW / MEANA: Fiji & Iraq.
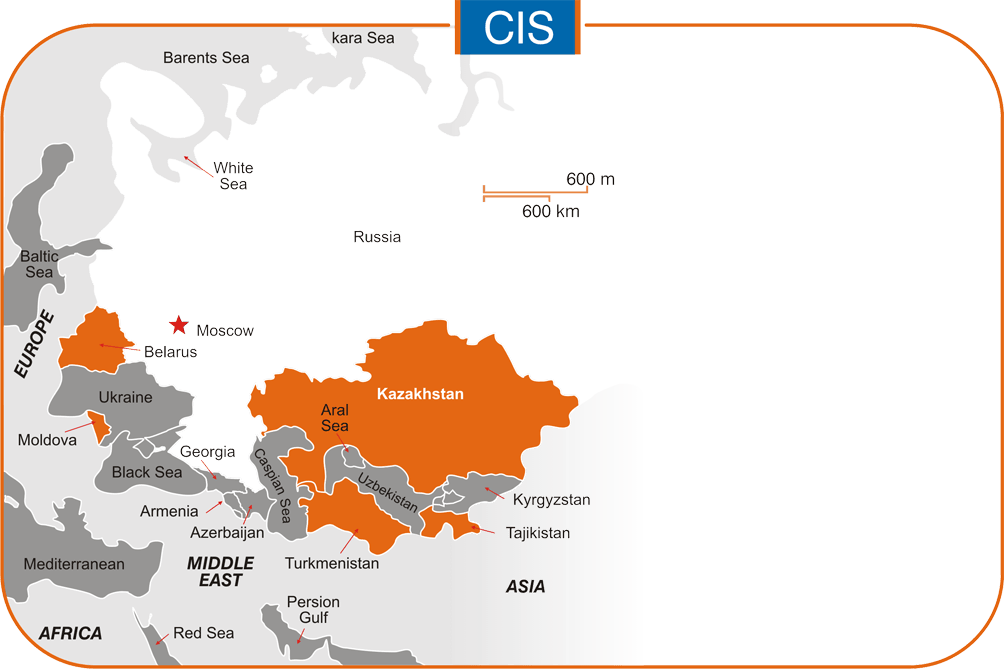
Asia: Afghanistan, Myanmar, Philippines, Turkmenistan, Uzbekistan & Vietnam.
We believe in working together towards success, with mutual trust, understanding our partner’s requirements and above all satisfying them.
Our Regulatory team consists of elite, highly qualified personnel engaged in providing data required to assure the high Quality, efficacy and safety of our Products. We can provide, clinical Trials, FDA Certificates and BE Studies for registration of our products as per guidelines of most health authorities in most countries.
We understand that distribution of pharmaceutical products must be undertaken with a great deal of care and attention. Raw materials awaiting processing and finished products awaiting dispatch are stored in modern, fully climate-controlled high-bay storage areas. We are able to meet the delivery needs of its customers thanks to its modern data and order processing systems, its inspections of all goods entering and leaving the factory, and the latest transport logistics (road, sea or air). No matter what route product takes, or how long it takes to get there, its journey is carefully monitored until it reaches its destination.
In a nutshell, the factors that have made us successful in drug manufacturing for overseas markets are:




